COVID-19 Vaccine FAQs
Here we answer some of the most common COVID-19 Vaccine Questions
We know there are a lot of questions out there about the COVID-19 Vaccine.
Please take a look at some of these most frequently asked questions and if you do not see yours, please call your Primary Care Provider for an answer!
Will the COVID-19 vaccine help prevent me from getting COVID or spreading it?
Yes, that is the intent. Vaccines are a simple, safe, and effective way of protecting people against harmful diseases before they come into contact with them.
Vaccinations such as the COVID-19 vaccine train your immune system to create antibodies just like it does when it’s exposed to any infection – granting immunity to the virus without ever being infected.
The vaccine has shown up to 90% effectiveness but we must continue to use every tool to stop this pandemic including vaccinations, hand washing, physically distancing, and wearing a mask.
Does Singing River Health System endorse the vaccine for its employees, patients, and community?
Singing River Health System fully supports and encourages all employees and patients to receive COVID-19 vaccines. Large-scale vaccination of our community is the best way to keep everyone safe and to end the COVID-19 pandemic.
Who is Eligible in Mississippi for the COVID-19 Vaccine?
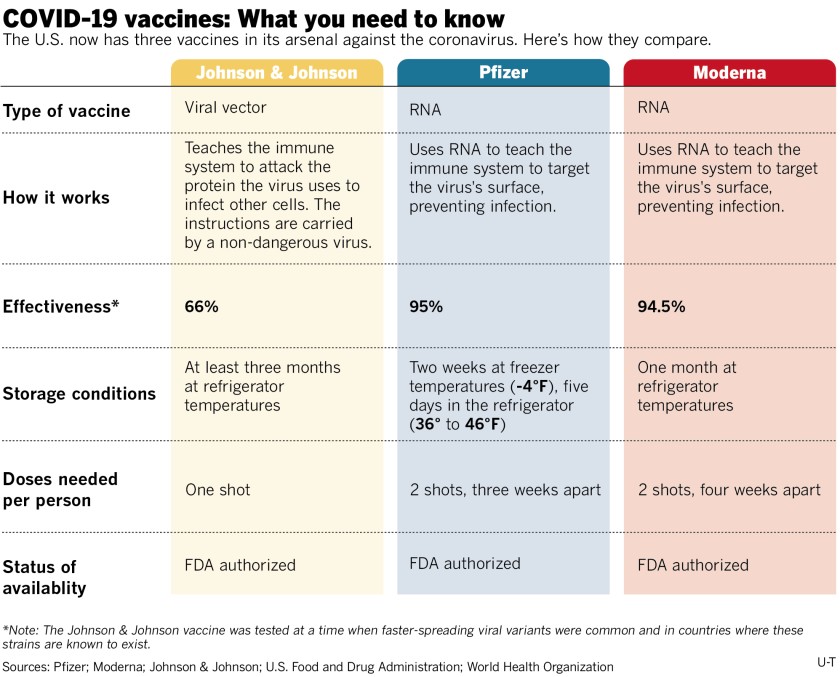
According to MSDH, eligible groups include ALL individuals age 12+ (Pfizer) and age 18+ (Moderna and Johnson & Johnson).
How do the Pfizer and Moderna vaccines work?
The messenger RNA (mRNA) found in the COVID-19 vaccine stimulates your body’s immune system to respond as if it were a true infection. The specifically designed mRNA teaches your cells how to mimic the viral proteins of COVID-19, triggering your immune system to create antibodies to fight it off.
If you are exposed to COVID-19 after receiving the vaccine, your body will automatically detect and destroy the virus before it causes severe illness. The vaccine will also minimize your risk of re-infection should you encounter or are exposed to the same virus again.
How does the Johnson & Johnson vaccine work?
The Johnson & Johnson vaccine does not use mRNA like Pfizer and Moderna. Rather, it’s an adenovirus vector vaccine, using an approach that employs a harmless cold virus to deliver a gene that carries the blueprint for the spiky protein found on the surface of the coronavirus.
The virus in the vaccine enters cells, which then follow genetic instructions to construct a replica of the coronavirus spike. The immune system uses these replicas to recognize and respond to the real thing, protecting you from COVID-19.
Will the vaccine give me COVID-19?
No. The mRNA technology in the Pfizer and Moderna vaccine does NOT use any live, dead, or weakened forms of viruses or bacteria to create antibodies. However, other vaccines that do contain strains of viruses like the Johnson & Johnson vaccine, do not cause the disease or put you at risk of its complications.
How safe and effective is the Johnson & Johnson vaccine?
Similar to the Moderna and Pfizer vaccines, it can cause sore arms, achiness, and mild fevers. Like the other COVID-19 vaccines, Johnson & Johnson is effective at preventing hospitalizations and deaths, including in South Africa against a more transmissible variant, and was 85 percent effective at protecting against severe cases of illness.
It was 72 percent effective at preventing moderate illness in the U.S. trials, a number that falls short of the Pfizer and Moderna vaccines, which were about 95 percent effective after both doses. However, experts say head-to-head comparisons among the vaccines cannot be made, because the trials were conducted at different times during the pandemic.
While reports of some clotting issues were seen in relation to receiving the Johnson & Johnson vaccine, it has proven overwhelmingly safe, with the CDC reporting that only 15 cases of this rare side effect had occurred as of April 23, 2021 out of more than 8 million doses given, and the “Known and potential benefits outweigh its known and potential risks.”
How many shots are required?
The COVID-19 vaccine will initially require one dose or two, depending on which vaccine you receive.
The two-dose vaccines (Pfizer and Moderna) will be given one dose at a time, after the first initial dose the individual will receive the second dose within 3-6 weeks after the first to ensure effectiveness.
Johnson & Johnson is a one-dose vaccine.
Just because some vaccines may have one shot or two, doesn’t mean that one is less effective than the other.
What happens if I don’t get the second shot within the allotted timeframe? Will I still be considered vaccinated?
To ensure optimal effectiveness, it is highly recommended that you receive the second dose within the specified timeframe as directed by the manufacturer. If you don’t receive the second shot, you will greatly decrease the chance of the vaccine working. You will be considered partially vaccinated.
How much does the vaccine cost?
There will be no cost for the COVID-19 vaccine at this time.
If I’ve already had COVID-19 and recovered, do I still need to get vaccinated?
Yes. There is not enough information currently available to say how long after infection someone is protected from getting COVID-19 again. In order to stop this pandemic, we need to use every tool available which includes immunization, social distancing, handwashing, and wearing masks.
As soon as you are clear of COVID-19 symptoms, you should get vaccinated.
Should I take a COVID test before or do I need to be COVID-free before receiving the vaccine?
You do not need to take a COVID-19 test prior to receiving the vaccine. If you are COVID-19 positive, then it is recommended that you follow the CDC guidelines and isolate for 10 days to limit exposure to others. Once you are out of isolation and symptom-free, you are able to receive the vaccine.
How long will the vaccine’s protection last?
Since this a novel virus, we are still unsure how long the vaccine antibodies will last. We are closely monitoring the research as more data becomes available. Booster vaccinations may be recommended for all or some individuals in the future, but research is still developing.
Are the COVID-19 vaccines safe?
Yes. COVID-19 vaccination is safe and side effects from the vaccine are minor and temporary, such as a sore arm or mild fever. Allergic reactions are very rare. The benefits of vaccination greatly outweigh the risks, and many more illnesses and deaths would occur without vaccines.
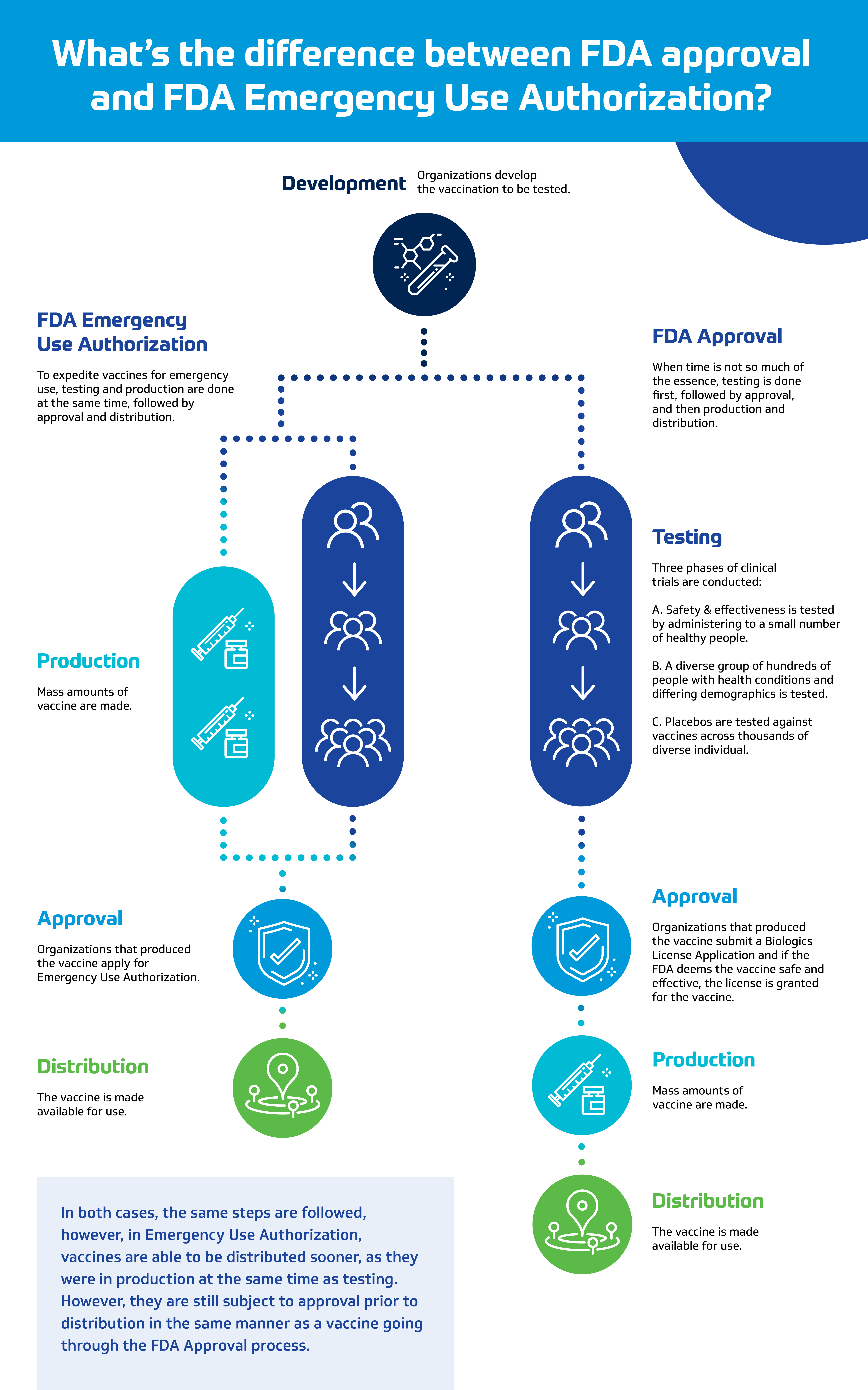
The FDA approval seems a bit rushed…
Even though the vaccine’s approval process was ordered at “warp speed”, before its release the FDA ensures any licensed vaccine is rigorously tested across multiple phases of trials before it is approved, including for emergency use. All vaccines are regularly reassessed once introduced and scientists are also constantly monitoring information from several sources for any sign that a vaccine may cause health risks.
“We are committed to expediting the development of COVID-19 vaccines, but not at the expense of sound science and decision making. We will not jeopardize the public’s trust in our science-based, independent review of these or any vaccines. There’s too much at stake.”
Stephen M. Hahn, M.D., FDA Commissioner, and Peter Marks, M.D., Ph.D., Director, Center for Biologics Evaluation and Research
Please take a look at this graphic, explaining the differences in the Emergency Use Authorization (EUA) process versus the traditional FDA approval process. Essentially, the main difference is the sequence of events. In an EUA process, production is done at the same time as testing, so once the trials are completed and the vaccine is deemed safe, it can be distributed more quickly.
On August 23, 2021, the Pfizer vaccine was given full FDA approval. Read more here about all of the data used to be approved by the agency.
Which vaccines are FDA approved?
At this time, the Pfizer vaccine is the only FDA-approved COVID-19 vaccine. It received approval on August 23, 2021.
See more about the FDA approval process for the Pfizer vaccine, or read a few key pieces below:
“Never before has the FDA has so much evidence to judge a shot’s safety.”
In approving the vaccine, “the Food and Drug Administration cited months of real-world evidence that serious side effects are extremely rare.”
“As for effectiveness, six months into Pfizer’s original study, the vaccine remained 97% protective against severe COVID-19. Protection against milder infection waned slightly, from a peak of 96% two months after the second dose to 84% by six months.”
The Johnson & Johnson and Moderna vaccines are being distributed under an Emergency Use Authorization, which calls for a high standard of safety and effectiveness (see previous question for a quick look at the difference between the two processes).